Engineering the Future of Life-Saving Medicines
Even as the COVID-19 pandemic began to subside in Austria, biotech company Biomay decided to invest in a new facility to manufacture essential protein- and DNA-based ingredients for the medicines of tomorrow. Just 25 months after the initial concept was developed, Biomay opened its new, state-of-the-art CDMO site near Vienna. Thanks to its development partners and the innovative concept behind the 4,000 m² high-tech facility, Biomay now operates a flexible manufacturing platform for a wide range of products in virtually any batch size.
Challenges and Solutions
Biomay faced several challenges, including increasing demand for diverse batch sizes and customized products. The production capacity of the older facility was limited, making it difficult to respond to market needs. With the support of VTU Engineering, Biomay developed a cutting-edge facility that overcame these limitations and significantly expanded its production capabilities. The new plant allows Biomay to scale up production and respond more quickly to changing market demands.

Client: Biomay AG
Location: Aspern Seestadt, Vienna, Austria
GMP Facility: Production of active ingredients based on proteins, DNA, and mRNA
Start-up: January 2022, 100 employees
GMP Manufacturing Area: 1,000 m² (1st floor)
Collaboration with VTU Engineering
The collaboration included feasibility studies, concept development, procurement, construction, and final certification. A special focus was placed on flexibility and sustainability. The facility is equipped with a groundwater heat pump for heating and cooling, and solar panels for fossil-free electricity. VTU coordinated the project and provided the expertise needed to meet the tight schedule.
Technical Details and Capacity
The new facility includes bioreactors with capacities of 5, 50, 150, and 750 liters and follows a classic GMP-compliant upstream-downstream production flow. A dedicated area is available for novel, patient-specific batches. VTU’s digital engineering platform supported efficient project organization and ensured compliance with all GMP certifications.
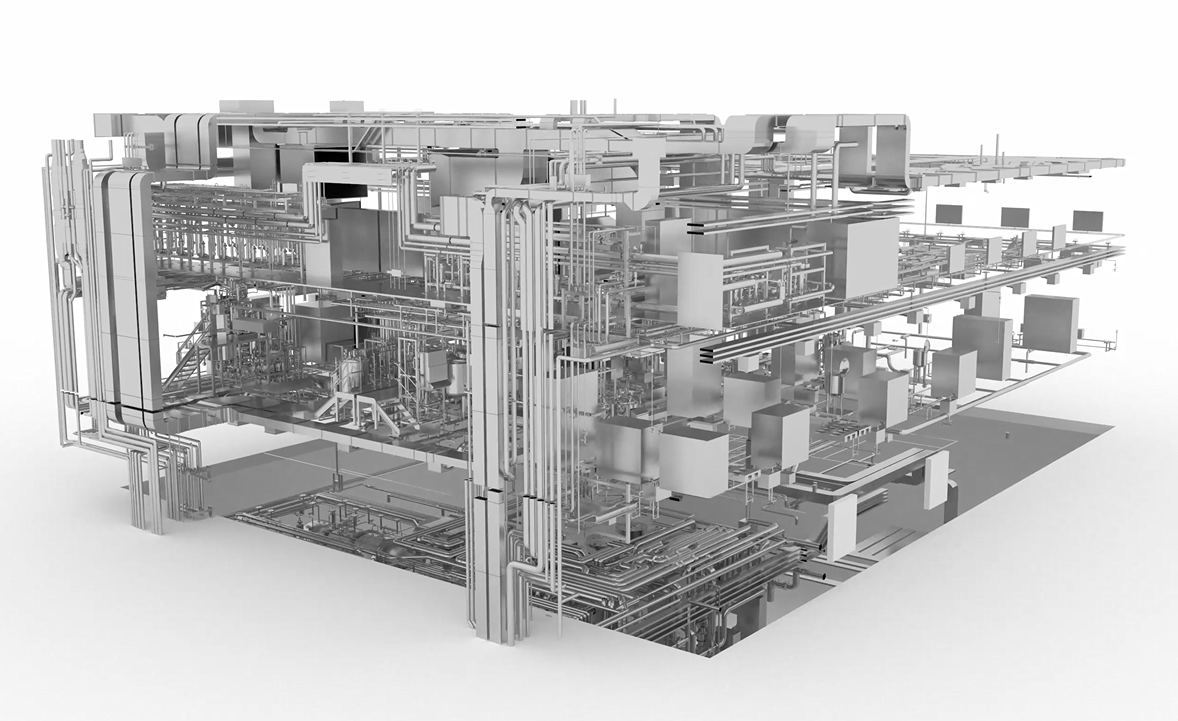
Success Factors
Key success factors included excellent collaboration, the use of advanced planning tools, and the ability to adapt flexibly to market needs. Despite the challenges of the COVID-19 pandemic, Biomay successfully completed and launched the facility. Planning and construction took 25 months, from late 2019 to January 2022. A broad range of competencies, digital tools, and out-of-the-box thinking were essential to the project’s success.

![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/d/b/csm_Biomay_Fotos_srgb-VPI4911_c137485673.jpg)